
Clinical
Studies
Proof of Concept (NL)
Company Initiated
Pathogen detection in breath of admitted Chronic Obstructive Pulmonary Disease (COPD) patients [n:15]
100%
Pathogen Detection in Breath (NL)
Company Initiated
Establishing causation of the lung infection in admitted Chronic Obstructive Pulmonary Disease (COPD) patients [n:55]
Succesfully Finished - Manuscript in Preparation
100%
Monitoring CF Patients (NL)
Investigator Initiated
Sampling Cystic Fibrosis patients for pathogen screening during routine visit [n:93]
Succesfully Finished - Peer-review Publication
100%
Contagiousness CoViD-19 (NL)
Investigator Initiated
Determining contagiousness of admitted patients with CoViD-19 [n:49]
Succesfully Finished - Peer-review Publication
100%
Multicentre Pathogen Detection in Breath (TZ)
Investigator Initiated
Diagnosing Community Acquired Pneumonia (CAP) in children visitting the hospital [n:>160]
Samples Collected
80%
Multicentre Monitoring CF Patients (NL)
Investigator Initiated
Sampling Cystic Fibrosis patients for pathogen screening during routine visit [n:>100]
Samples Collected
80%
Early Diagnosis Tuberculosis (SA)
Investigator Initiated
Diagnosing Tuberculosis in children visitting the hospital [n:>5]
Collecting Samples
25%
Patient-Friendly Sampling
for Fast Diagnosis
Better sample, Better treatment
SARS-CoV-2 RNA in exhaled air of hospitalized COVID-19 patients
L. Kurver, et al., Scientific Reports, 2022
Non-invasive diagnostics of pathogenic bacteria using a breath sampler in children with cystic fibrosis
K. J. van Aerde, et al., Journal of Breath Research, 2022
Peer-reviewed
Publications
Unambiguous Diagnosis is Hampered
by Conventional Sampling Methods
Better sample, Better treatment
White
Papers
Pathogen Detection in Exhaled Breath
Detecting bacterial pathogens in exhaled breath of admitted patients with Chronic Obstructive Pulmonary Disease
Exclusively Sampling the Lungs
Comparing viral-load of Aerocep with different compartments in the upper respiratory tract
Clinical Impact of Aerocep
Early detection Pseudomonas aeruginosa for irradication treatment in patients with Cystic Fibrosis
Clinical impact of aerocep-based diagnosis

Treatment Changing Results
Samples Lower Respiratory Tract
Patient-Friendly

AIM
To demonstrate the impact of the aerocep result on the treatment of patients with cystic fibrosis.
APPROACH
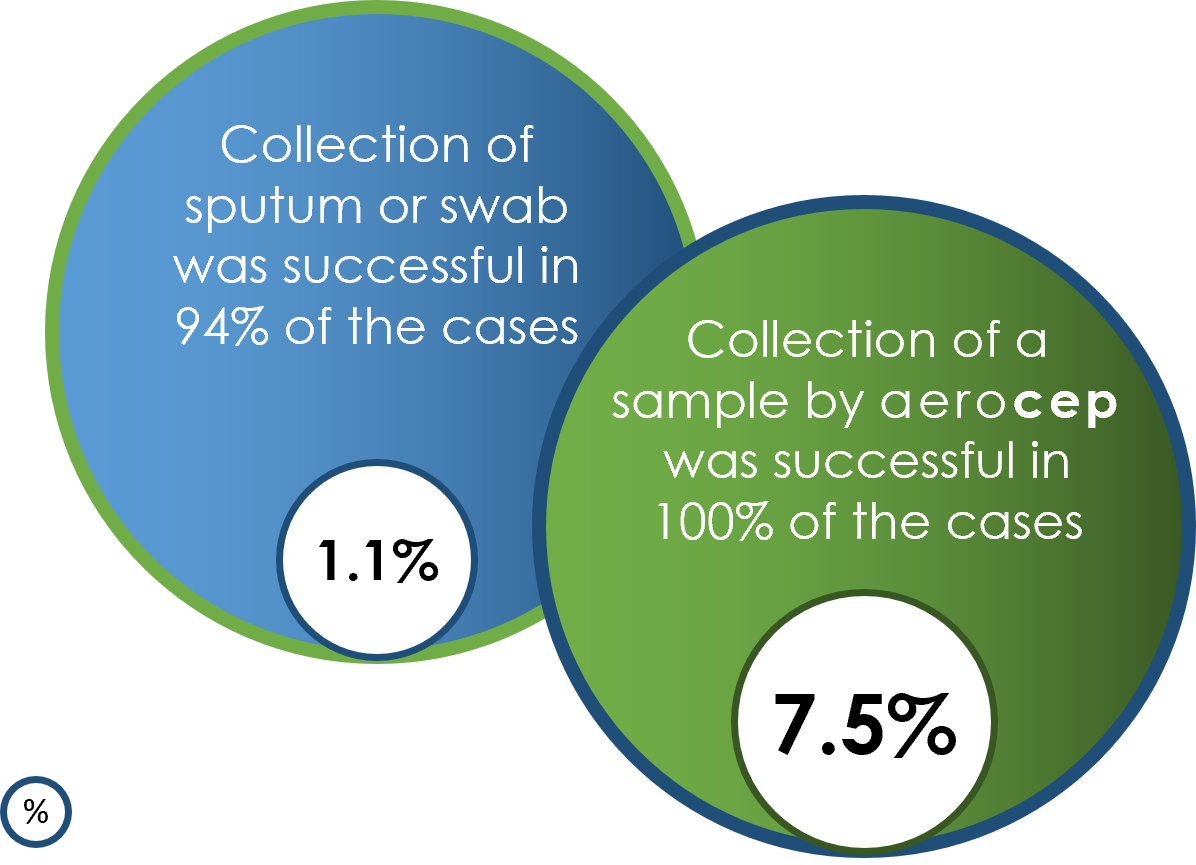
Sputum, swab and breath samples were collected from paediatric patients with cystic fibrosis (CF) to detect airway pathogens, including Pseudomonas aeruginosa an important pathogen which needs to be detected in an early phase to allow immediate treatment to prevent chronic lung damage. We further assessed the patient-friendliness of the sampling method by interviewing the paediatric patients and their parents.
RESULTS
Results between conventional and exhaled breath sampling differed significantly: S. aureus, a frequent colonizer of the upper respiratory tract, was found more often in swab or sputum samples whereas P. aeruginosa known to reside in the lower respiratory tract of CF patients was found more often in breath samples. The figure below displays the comparison of the results between conventional and aerocep-based sampling.
CONCLUSION
Breath samples collected by the aerocep show a higher sensitivity for the detection of P. aeruginosa. These results confirm that the aerocep samples the lower respiratory tract. Furthermore, this shows promise to be applied for early treatment of CF patients. In addition, most patients and their parents experienced the aerocep-based sampling as less burdensome than conventional sampling.
Origin of Data
‘Non-invasive diagnostics of pathogenic bacteria using a breath sampler in children with cystic fibrosis’, K. J. van Aerde, et al., Journal of Breath Research, 2022.
AIM
To determine whether the aerocep samples only or primarily the lower respiratory tract.
APPROACH
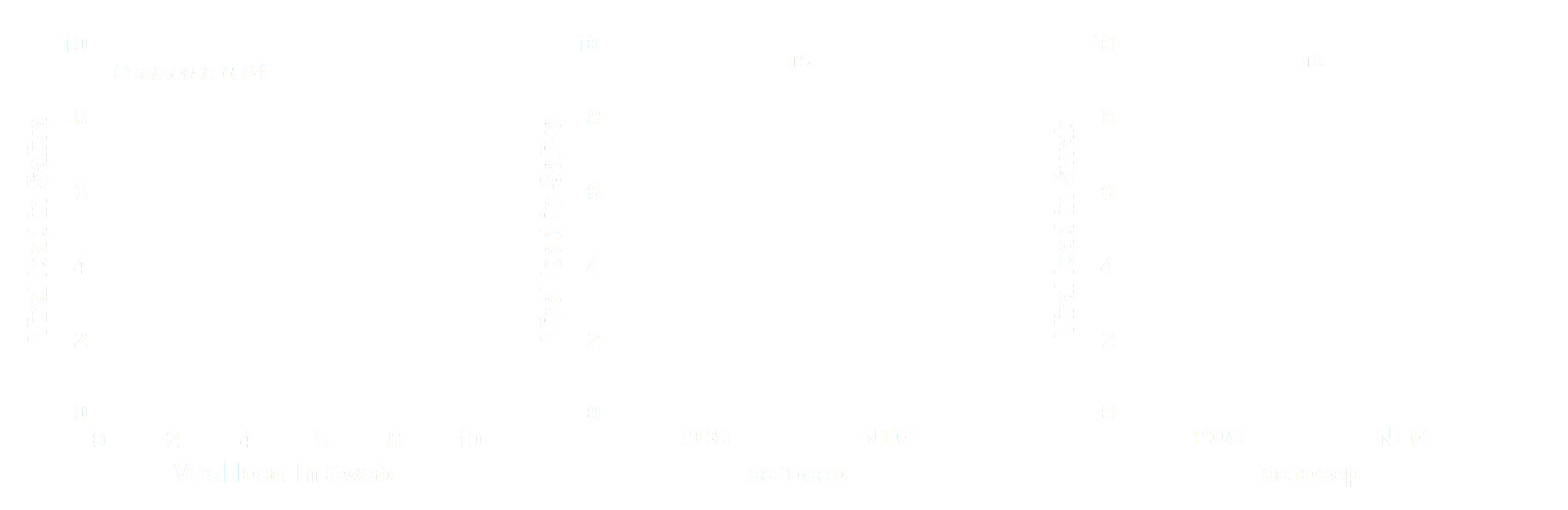
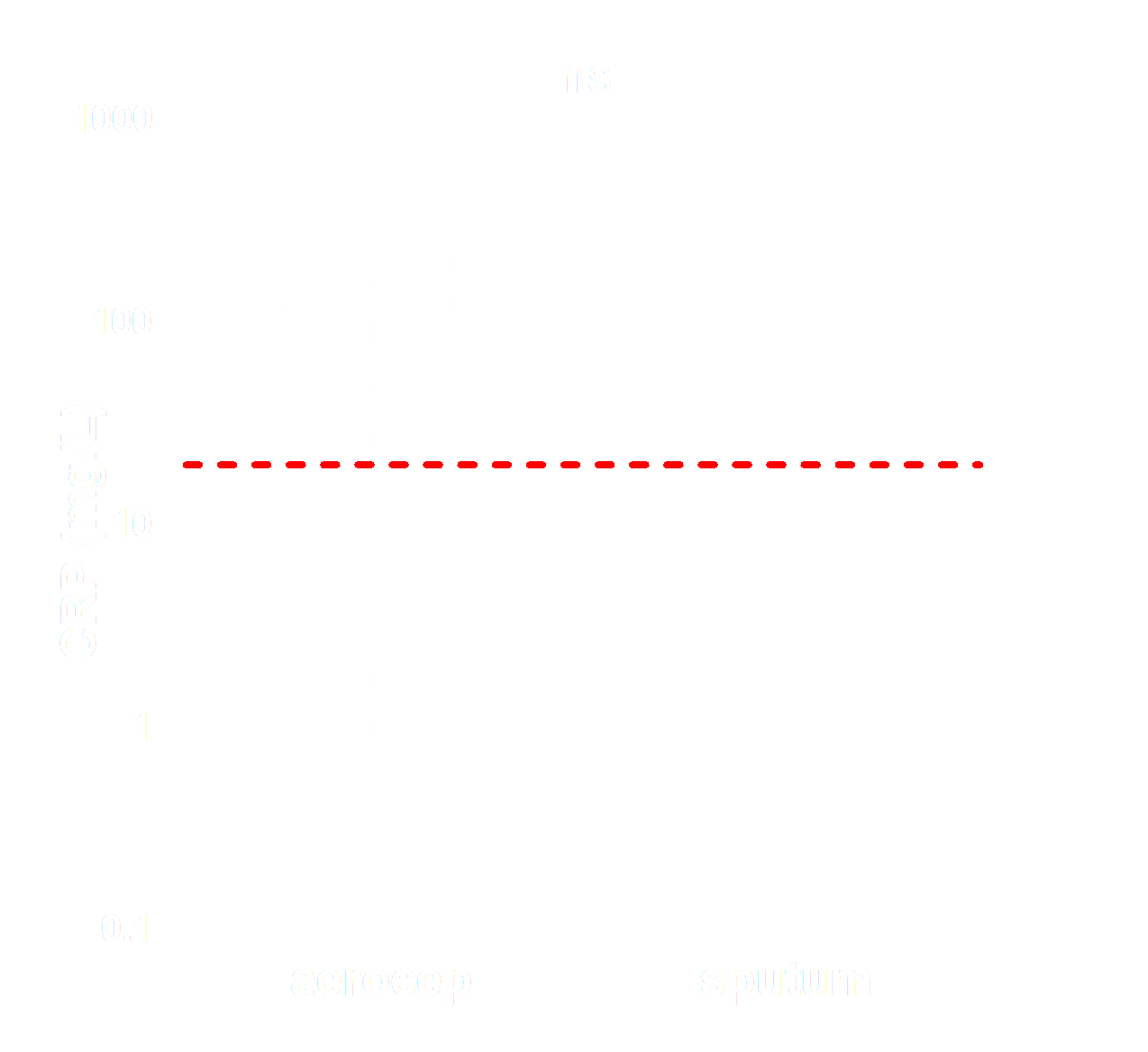
Saliva, nasopharyngeal swabs, and breath samples were collected from hospital admitted COVID-19 patients. The viral load in the different sample types was determined and compared to assess whether the breath sample can be distinguished from the upper respiratory tract samples (swabs and saliva). We assumed that high correlations of viral loads between swab samples or saliva and breath samples would suggest contamination from the upper respiratory tract, while low correlations would indicate that the breath is derived from the lower respiratory tract.
RESULTS
The viral load of the breath samples did not correlate with saliva nor nasopharyngeal swabs. The figures below display the viral load for each sample and the correlation between viral load in these respective samples. Saliva and swab display a good correlation, while samples drawn by the aerocep do not show anycorrelation with saliva or swab.
CONCLUSION
The viral load of aerocep samples does not correlate with nasopharyngeal swabs and saliva samples. We conclude that the aerocep has proven successful in measuring aerosols from the lower airways loaded with SARS-CoV-2, herewith contagiousness.
Origin of Data
‘SARS-CoV-2 RNA in exhaled air of hospitalized COVID-19 patients’, L. Kurver, et al., Scientific Reports, 2022.
Confirmation that only lower airways are sampled
Samples Lower Respiratory Tract
Measure Contagiousness
Patient-Friendly

Feasibility of pathogen detection from exhaled air

Detecting Pathogens
Clinically relevant
Patient-Friendly

AIM
To detect bacterial pathogens in exhaled air collected by the aerocep by molecular diagnostics and validation of the clinical relevance.
APPROACH
This study contains two phases; a pilot and proof-of-concept phase. Pilot study: Assessment of breath samples from 9 hospital admitted COPD patients to determine whether any potential pathogen could be measured in exhaled air. Proof-of-Concept study: Collection of breath samples from 46 admitted COPD patients to compare results with sputum culturing and c-reactive protein (CRP) in serum. In both studies the patient experience with this innovative way of sampling was assessed using questionnaires.
RESULTS
Pilot study: In 6 out of 9 breath samples relevant bacterial pathogens were detected, e.g., H. influenzae, S. pneumoniae and P. aeruginosa. Proof-of-Concept study: Sputum culturing led to conclusive results in 22% of the cases, whereas 52% breath sample tested positive for relevant pathogen. Most of the sputum and aerocep positive patients showed elevated CRP levels in serum, associated with bacterial infection, confirming the relevance of the aerocep results.
CONCLUSION
Pilot study: Breath samples drawn by the aerocep can be used for pathogen detection by molecular diagnostics. Proof-of-Concept study: aerocep-based pathogen detection is more sensitive than sputum-based detection, 52% vs 22%. In addition, the breath sample is clinically relevant as it correlates with CRP in serum. In addition, positive feedback was received from patients who used the aerocep.
Origin of Data
Pilot and Proof-of-Concept studies 2019; Assessment of COPD patients admitted with an exacerbation (unpublished).
